1. About SI-ATRP Controlled/“living” surface-initiated radical polymerization offers the possibility to generate brush like polymeric thin films with controllable thickness, composition, and architecture. Polymer brushes have found widespread applications as model responsive and non-biofouling surfaces, in protein binding and immobilization studies, chromatography supports, in membrane applications, antibacterial coatings, actuation, and low friction surfaces et. al. Among the many controlled polymerizations, atom-transfer radical polymerization (ATRP) is most widely used, particularly for surface grafting in aqueous solution are attractive from both economic and environmental points of view. In theory, SI-ATRP can be carried out on any substrates if one can immobilize the ATRP initiator on the desired surface.
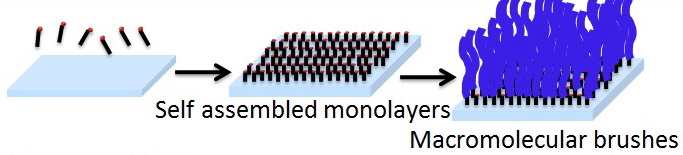
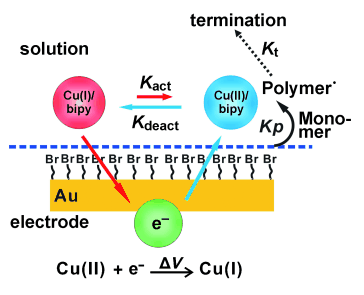
2.New method for SI-ATRP : a. SI-eATRP on conducting surfaces such as gold
ATRP initiator formation: Gold: The homogeneous SAMs of thiol ester initiator ω-mercaptoundecyl bromoisobutyrate were prepared by immersing the gold-coated silicon wafer (electron beam evaporation of 100 nm thick gold on 5-7 nm thick chromium) into 2 mM ethanol solution for 12 h at room temperature. The patterned, initiator-terminated thiol monolayer (with a bare gold background) was prepared by micro-contact printing (μCP) with a patterned PDMS stamp.1 The wafers modified with SAMs were thoroughly rinsed with ethanol to remove the physisorbed initiator and then dried in a stream of nitrogen. Polymerization: Prior to each polymerization, CVs measurements were carried out using a platinum wire counter electrode, SCE reference electrode and initiator modified gold-coated wafer working electrode. eATRP was initiated at a constant potential (i.e. the reduction potential of copper(I)) and continued for a certain time at room temperature to yield the polymer brushes modified electrode. Monomer solutions can be reused many times.2
b. SI-eATRP on non-conducting surfaces
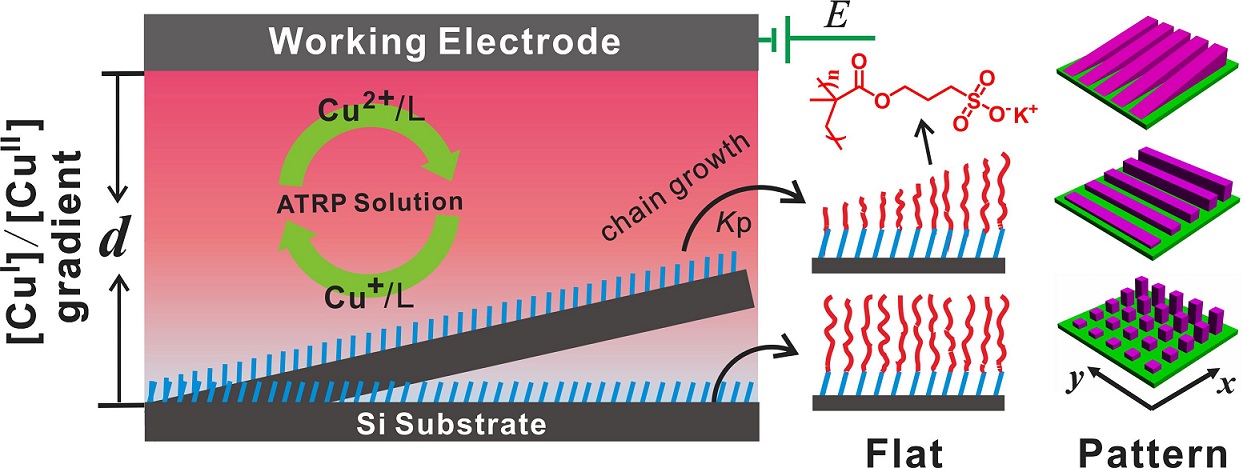
Schematic Illustration of Using Diffusion to Control eATRP for Surface Modification Initiator mobilization: Silicon or PDMS: Plasma oxidised clean silicon substrate and PDMS were placed in a vacuum desiccator with a vial containing 5 μL of 3-(trichlorosilyl)propyl 2-bromo-2-methylpropanoateinitiator. The chamber is then pumped down to <1 mbar, isolated from the pump and left under vacuum for 30 minutes and then cycled for 3 times.
Others: Catechol-based adhesive compounds can be bonded to versatile material surfaces at high binding strength, including noble metals, native metal oxides, semiconductors, polymers, ceramics, which represents a very promising and biomimetic approach to extend the field of surface functionalization.4 Polymerization: The eATRP was carried at constant applied potential with the same three-electrode system in the same solution as above, except that an initiator modified substrate was placed in counterpart position of the working electrode and separated by a U shape silicone elastomer membrane. The distance between working electrode and substrate can be adjusted by the thickness of silicone membrane, while the tilting angle was adjusted by two separate membrane of different thickness. CuI/bipy activator was generated at the platinum working electrode under a constant potential, eATRP was initiated when CuI/bipy diffused to the initiator modified substrates faced to platinum working electrode and continued for a certain time at room temperature to yield polymer brushes.3 c. UV-ATRP (Ultraviolet Light-Induced Surface-Initiated Atom-Transfer Radical Polymerization) 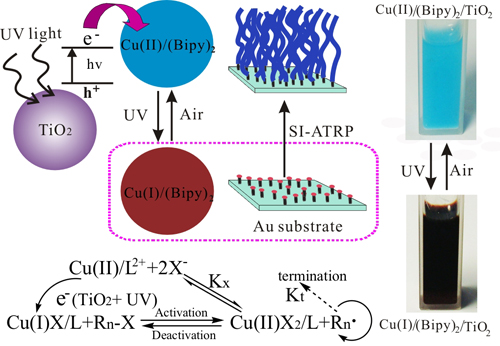
UV light-induced surface-initiated atom-transfer radical polymerization (UV-ATRP) uses TiO2 nanoparticles as photoactive materials to reduce Cu(II)/L to a Cu(I)/L complex under UV irradiation by a one-electron transfer process for ATRP with multiple usage of monomer solutions. The growth of polymer brushes can be manipulated by either varying the content of photoactive materials or regulating the irradiation intensity, thereby yielding polymer brushes with controllable thickness, composition, and architecture.5 d. sa-ATRP (Sacrificial-Anode Atom-Transfer Radical Polymerization) 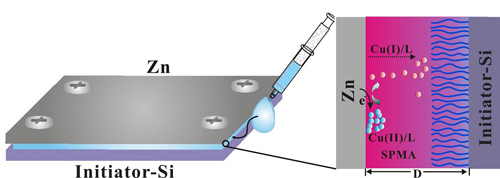
Electrochemical potential difference allows surface initiated ATRP occurring in a metal-substrate sandwiched architecture in air using volumes as small as 10 µL, in which CuI activators are continuously generated and diffused to initiator modified substrate, while the presence of CuII deactivators benefit to keep polymerization living for even longer time. Polymer brushes gradients plus complex shapes can be easily obtained due to spatially distribution of catalysts. The unique advantage of the novel approach is to carry out polymerizations of multiple monomers on a single surface very easily and theoretically, many polymer brushes can be formed easily if more template wells/channels are prepared and monomer solutions can be delivered in each.6 References -
Feng Zhou, Zijian Zheng, Bo Yu, Weimin Liu, Wilhelm T. S. Huck, Multicomponent Polymer Brushes, J. Am. Chem. Soc. 2006, 128, 16253-16258. -
Bin Li, Bo Yu, Wilhelm. T. S. Huck, Feng Zhou, Weimin Liu, Electrochemically induced surface-initiated atomic transfer radical polymerization, Angew. Chem. Int. Ed, 2012, 51, 5092-5095. -
Bin Li, Bo Yu, Wilhelm T. S. Huck, Weimin Liu, Feng Zhou, Electrochemically mediated atom transfer radical polymerization on non-conducting substrates: controlled brush growth through catalyst diffusion, J. Am. Chem. Soc., 2013, 135, 1708-1710. -
Jianxi Liu, Qian Ye, Bo Yu, Xiaolong Wang, Feng Zhou, Contact printing biomimetic catecholic monolayer on a variety of surfaces and derivation reaction, Chem. Commun., 2012, 48, 398–400. -
Junfeng Yan , Bin Li , Feng Zhou *, and Weimin Liu, Ultraviolet Light-Induced Surface-Initiated Atom-Transfer Radical Polymerization, ACS Macro Lett., 2013, 2, 592–596. -
Junfeng Yan, Bin Li, Bo Yu, Wilhelm T S Huck*, Weimin Liu*, Feng Zhou*, Controlled Polymer-Brush Growth from Microliter Volumes using Sacrificial-Anode Atom-Transfer Radical Polymerizationfrom, Angew. Chem. Int. Ed., 2013, DOI: 10.1002/anie.201304449
|